Toxicology and Radiation Biology
- Toxicology
- Biodosimetry
- Environmental Mutagenesis
- Mutation Research
Based on Metafer operated scanning systems, MetaSystems offers an unrivaled portfolio of imaging automation applications for toxicology, radiation biodosimetry, and mutation research. The Metafer software is the perfect tool for dose-response analyses – extremely fast to generate instant results in biodosimetry, fully compliant to GLP regulations applied to preclinical genotoxicity studies, sensitive to detect even low dose effects in environmental mutagenicity tests, and highly flexible to be adapted to any study in mutation research labs.
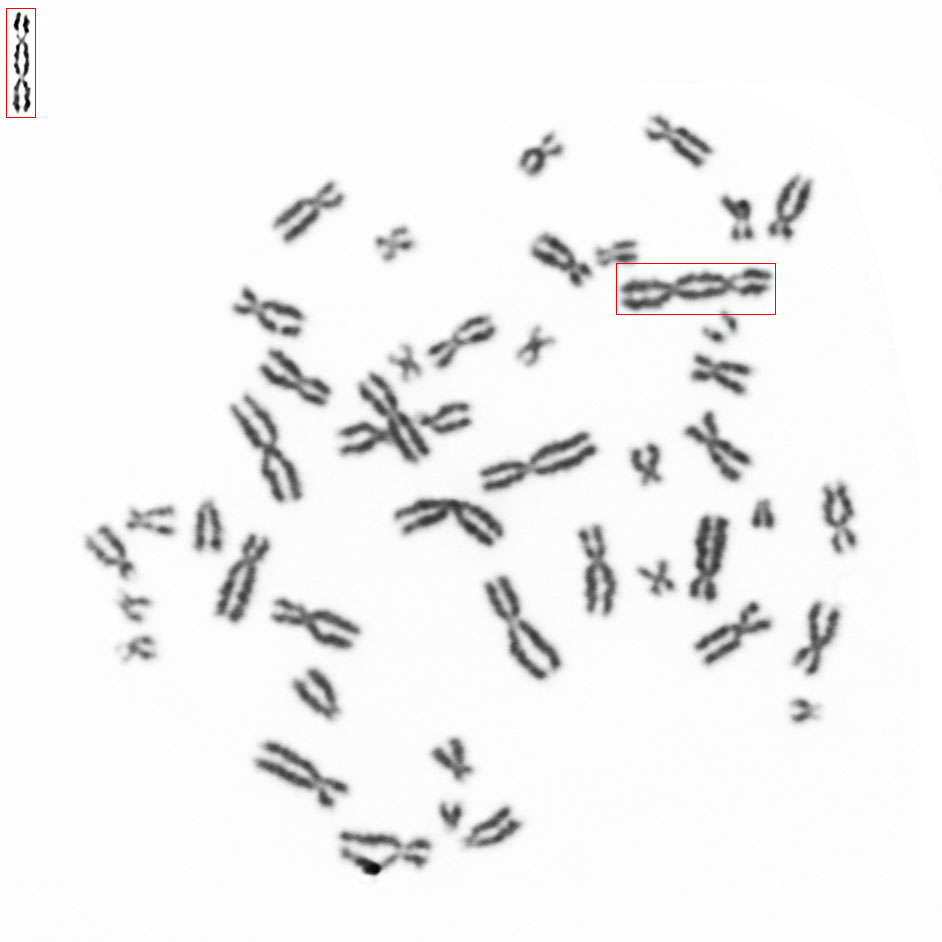
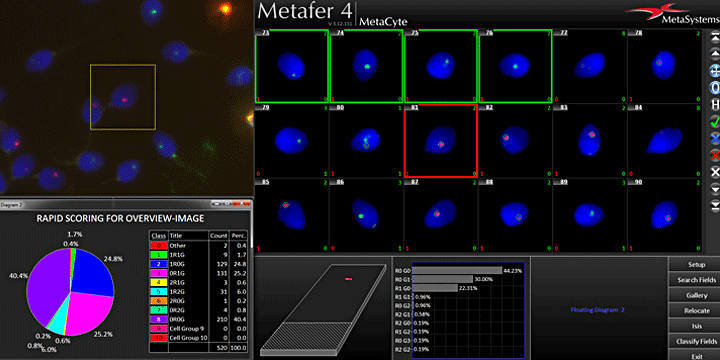
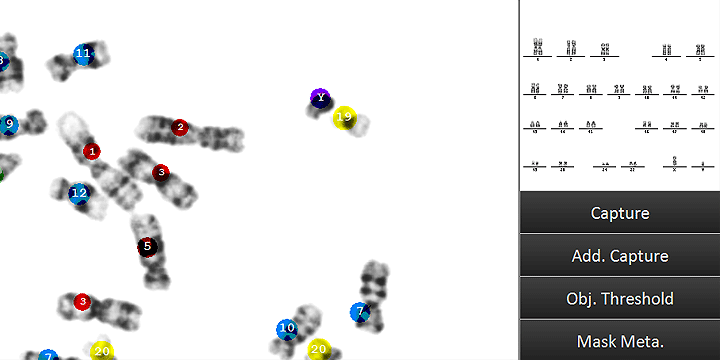
Counting dicentric chromosomes and other chromosomal aberrations is generally referred to as the 'Gold Standard' for radiation biodosimetry. Though the assay has been proven to deliver best dose estimates in radiation accidents and in toxicology studies, it is usually time-consuming and labor-intensive. The Metafer operated smart scanning with its dedicated tools and the automated dicentrics scoring software module Metafer DCScore frees the researcher from all tedious and error-prone tasks on the way. Additionally, it offers the complete working environment to generate fully documented and reliable results in the shortest time – of course compliant to GLP regulations and to the OECD guideline #473 (In-Vitro Mammalian Chromosome Aberration Test).

The cytokinesis-block micronucleus (in-vitro MN) test and the mammalian erythrocyte micronucleus (in-vivo MN) test are both used as tools for fast and precise quantification of DNA damage. The Metafer operated scanning offers complete automation for both tests – reliable, fast, and fully documented. Users of the in-vitro MN test receive their results in a few minutes, and each detected bi-nucleate is displayed as a gallery image, together with its micronucleus count in the Metafer MNScoreX module. The amounts of mono-, bi-, and multi-nucleates are recorded and can be used to calculate the CBPI.
For the in-vivo MN test, the Metafer MetaCyte module automatically assigns each cell to its respective sub-population (PCE or NCE). The assignment is done based on the staining of the cell, and it has the robustness to automatically adapt to various staining conditions. Micronuclei are counted in each population and displayed separately as a histogram. Both tests are GLP compliant and follow the directive of the respective OECD guidelines (#487 for the in-vitro MN test and #474 for the in-vivo MN test). Results can either be summarized in comprehensive and customizable reports, or directly be exported to any external statistics software package.
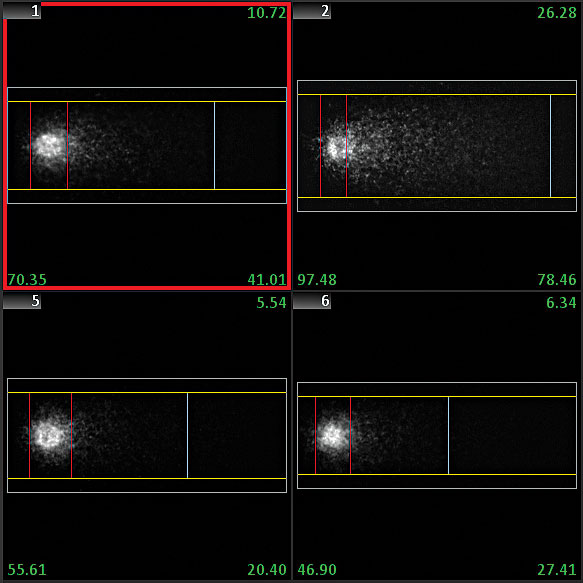
The Comet assay, also known as single cell gel electrophoresis (SCGE) assay, detects DNA damage and repair in individual cells. One major advantage of the Comet assay is the direct assessment of damage on a cell-by-cell basis. The sensitivity of the assay, however, makes it vulnerable to various factors that can affect reproducibility of results.
Users of MetaSystems' smart scanning solutions are able to fully standardize the analysis part of the Comet assay. MetaSystems offers Metafer modules to operate an interactive manual imaging system or an automated slide scanning system.
Sophisticated software algorithms in Metafer CometScan obtain head and tail intensities, lengths, and background conditions, and they automatically calculate tail moments and Olive tail moments. The automated system based on the Metafer software performs the analysis completely unattended, and it is capable to distinguish between ‘normal’ cells and hedgehog comets. Each cell is documented as an image and can also be re-located on the microscope. Data are displayed in handy reports, on-screen graphs, and values assigned to the gallery images.
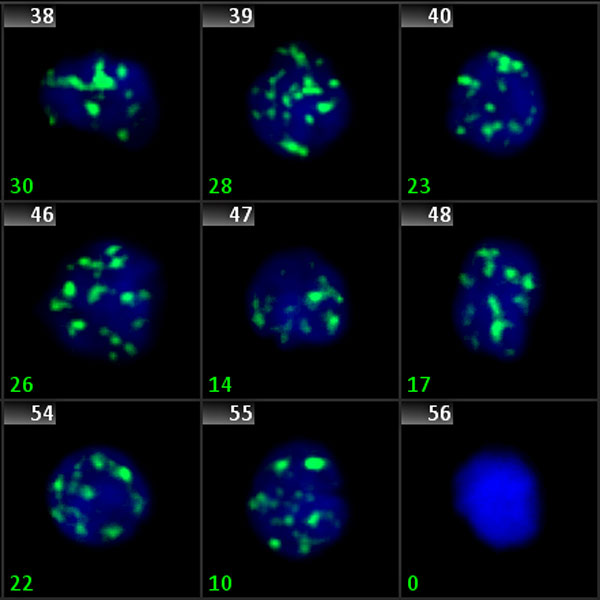
Phosphorylated H2AX (also known as γ-H2AX) is a marker for DNA double strand breaks. Fluorescently labeled γ-H2AX protein clusters can be microscopically detected, and thus, can be used as a very direct marker for DNA damage and repair. The Metafer operated scanning system finds target cell nuclei, acquires signals from multiple focus planes and in up to 12 color channels, and automatically proposes an evaluation of the signals. Co-localization of signals from different channels, analysis of fused signals, and intensity measurements are possible.
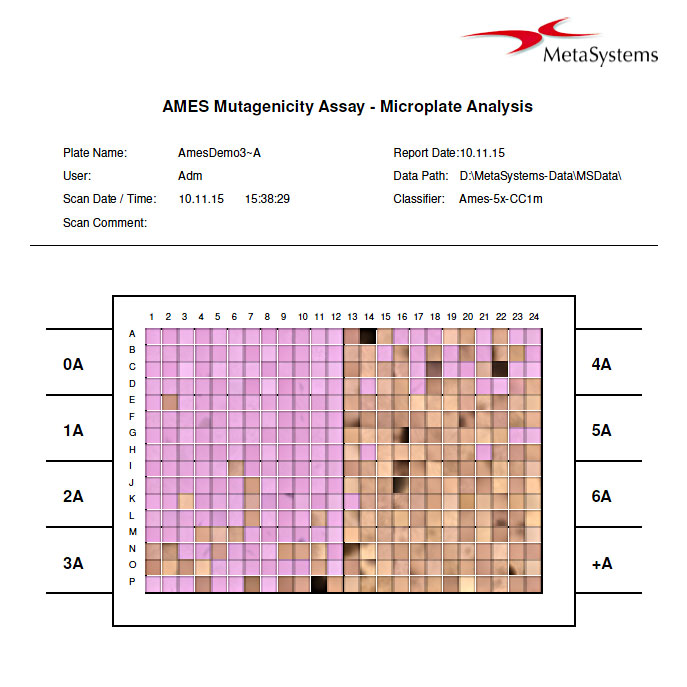
The Ames II / Ames MPF test (based on the method of Xenometrix AG, Basel, Switzerland) is widely used to assess the mutagenic and toxic potential of compounds. The test uses bacteria carrying DNA mutations in genes involved in the synthesis of the amino acid histidine. The Ames II and Ames MPF assays are modernized versions of the original Ames test, using liquid culture instead of agar plates. The assays are done on 384-well micro titer plates and have a colorimetric read-out, which can be automatically evaluated in Metafer MetaCyte. Each single well is interpreted either positive or negative, giving the user the opportunity to change automatic scores in case of ambiguous results. Printed results contain color images of each well resulting in a complete overview of the 384 well micro plate.
Metafer 4.3 and Ikaros 6.3 are classified as in vitro diagnostic medical devices (IVD) in the European Union in accordance with In Vitro Diagnostics Regulation (EU) 2017/746 or In Vitro Diagnostic Medical Device Directive 98/79/EC, respectively, and carry the CE label unless otherwise indicated. Use all MetaSystems IVD products only within the scope of their intended purpose.
MetaSystems products are used in many countries worldwide. Depending on the regulations of the respective country or region, some products may not be used for clinical diagnostics.
Some hardware components supplied by other manufacturers are not included in MetaSystems IVD products and are therefore not IVD medical devices.
Please contact us for further information.