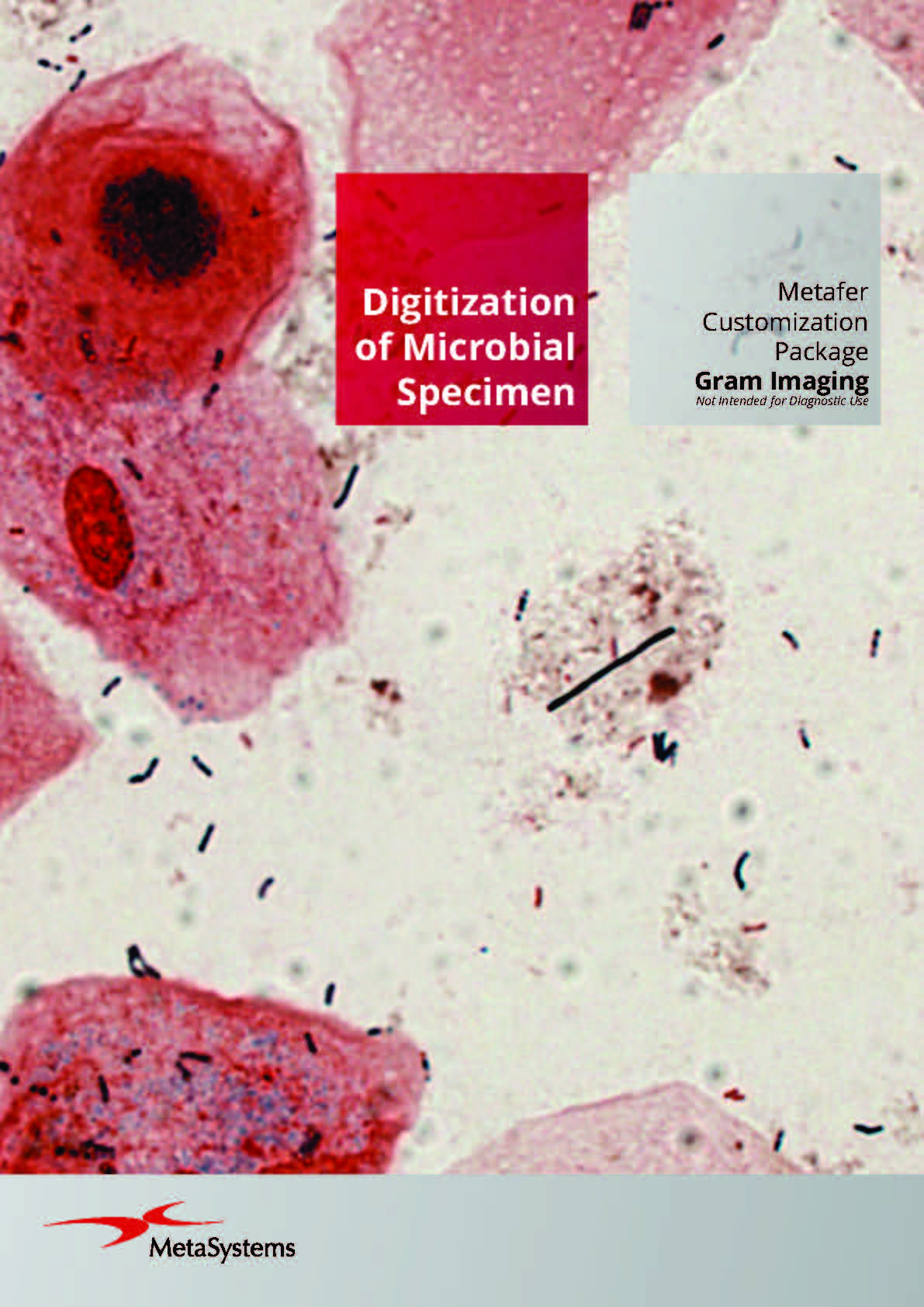
Metafer
The versatile Metafer slide scanning software offers automation for various imaging applications in life sciences microscopy.
Metafer is a software intended to control microscope and accessory hardware, to acquire digital images, and to assist the operator in the detection, classification, and counting of cells of human or other origin and other objects in microscopic specimen.
Metafer is intended for use in in vitro diagnostic procedures by clinical and non-clinical laboratories in accordance with their established procedures. Slide scanning and analysis conditions can be adapted to a variety of specimen, including, but not limited to, cultured and stained cells in their interphase or metaphase state. The analytical and clinical performance has not been established.
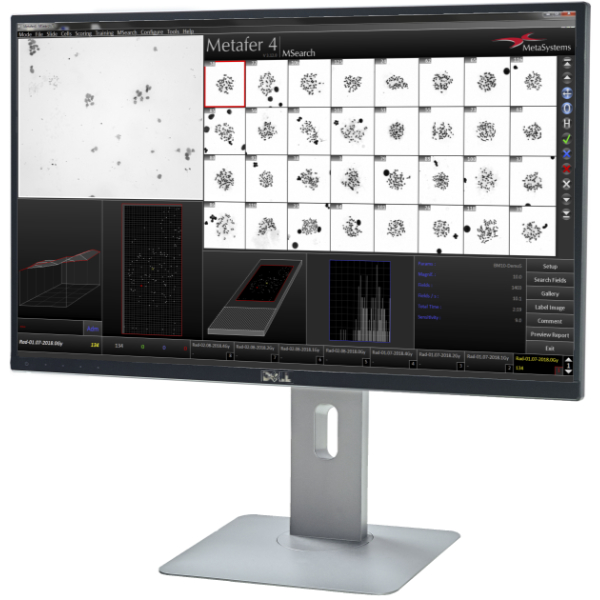
- Capture up to 800 slides seamlessly in a single session.
- Utilize various contrasting methods, including but not limited to brightfield, fluorescence, phase contrast, darkfield, and more.
- Effortlessly link and combine different imaging tasks, letting Metafer engage the objectives, and benefit from an automated immersion oil dispenser.
- Enable barcode reading for specimen identification and tracking, allowing Metafer to capture images from the frosted end of the specimen.
- Take advantage of a range of settings to establish automated focusing tailored to the specimen's specifics.
- Review all imaging results in a well-organized image gallery.
- Efficiently relocate identified objects on the microscope with a single mouse click.
- Customize workflows according to your preferences using a flexible classifier-based imaging approach.
- Expand your imaging network by integrating multiple workstations managed by Metafer and Ikaros.
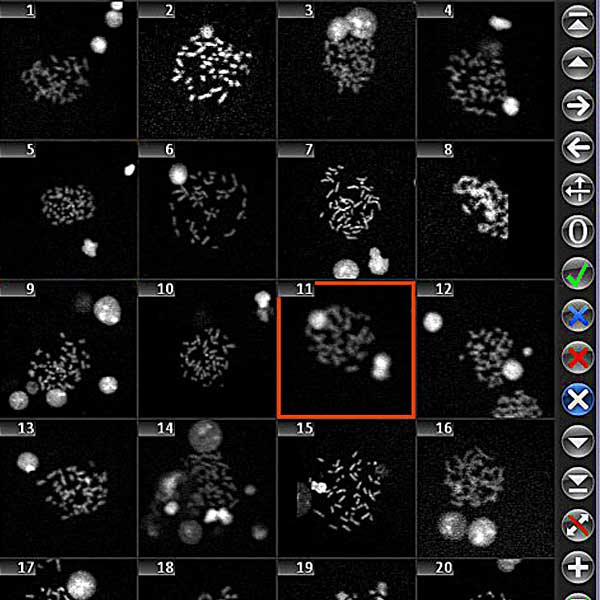
Utilizing the Metafer software, imaging systems scan specimens of diverse sizes, employ various contrasting methods and magnifications, and identify, classify, and enumerate cells or other objects. Metafer's modularity and flexible architecture make it proficient in assisting users from various fields with their specific imaging tasks.
In contrast to complex analysis systems that can be unwieldy and challenging to navigate, Metafer's intelligent scanning offers a different experience: unhindered access to all data, a user-friendly display of results, and complete control over imaging parameters to ensure precise results according to your preferences. With Metafer, slide scanning becomes remarkably straightforward.
Given the variability of each microscopic sample, automated imaging demands flexibility. Metafer's distinctive concept provides an effective solution: user-trainable classifiers accurately establish imaging standards, and by selecting the relevant classifier, the integrated scanning system swiftly transitions to the next task.
MetaSystems solutions are adaptable to your expanding requirements. The modular software and supported hardware allow for increased automation as the workload grows. Additional accessories like the SlideFeeder x80, a barcode reader, and an automatic immersion oil dispenser can be seamlessly incorporated as needed.
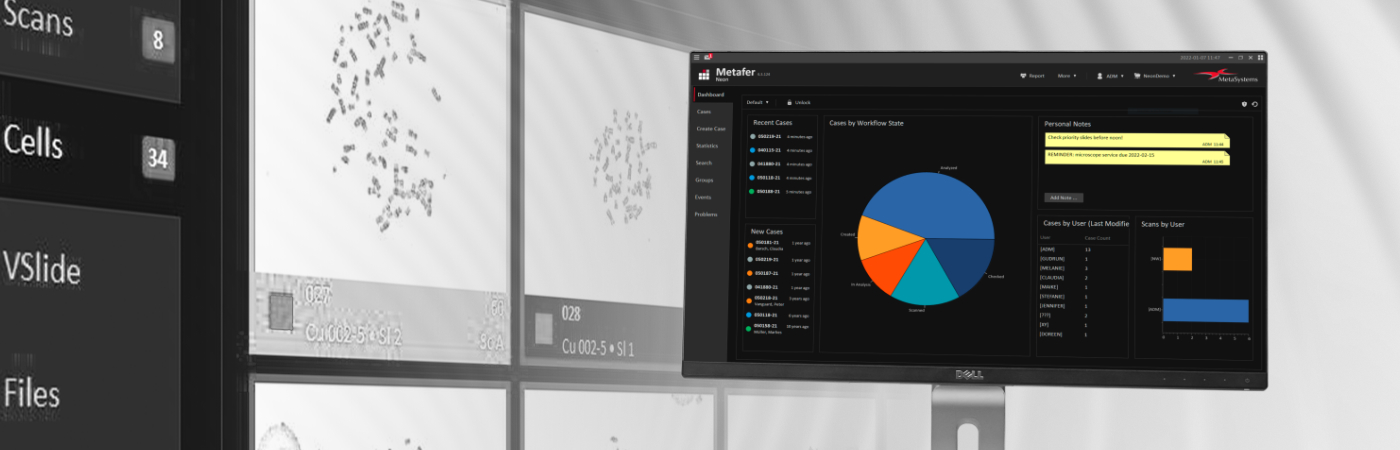
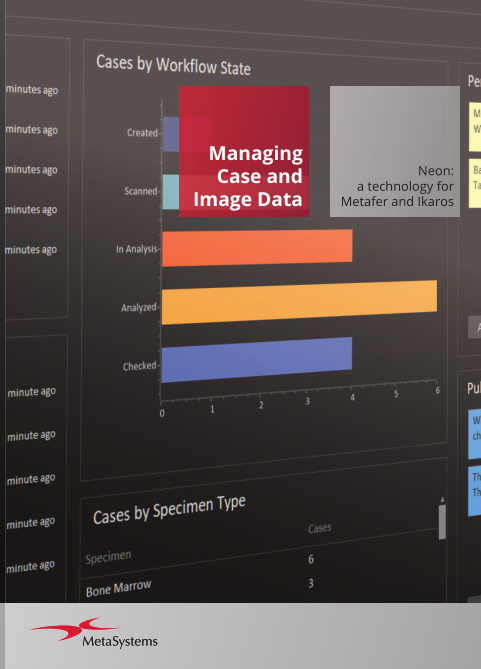
Neon is a convenient interface for organizing case and image data. Users configure overview dashboards to summarize cases, processing statuses, and case statistics. Interfaces for data exchange with external databases can be adapted. The fields on case data sheets and reports are customizable to meet specific needs.
Neon serves as general data management software.
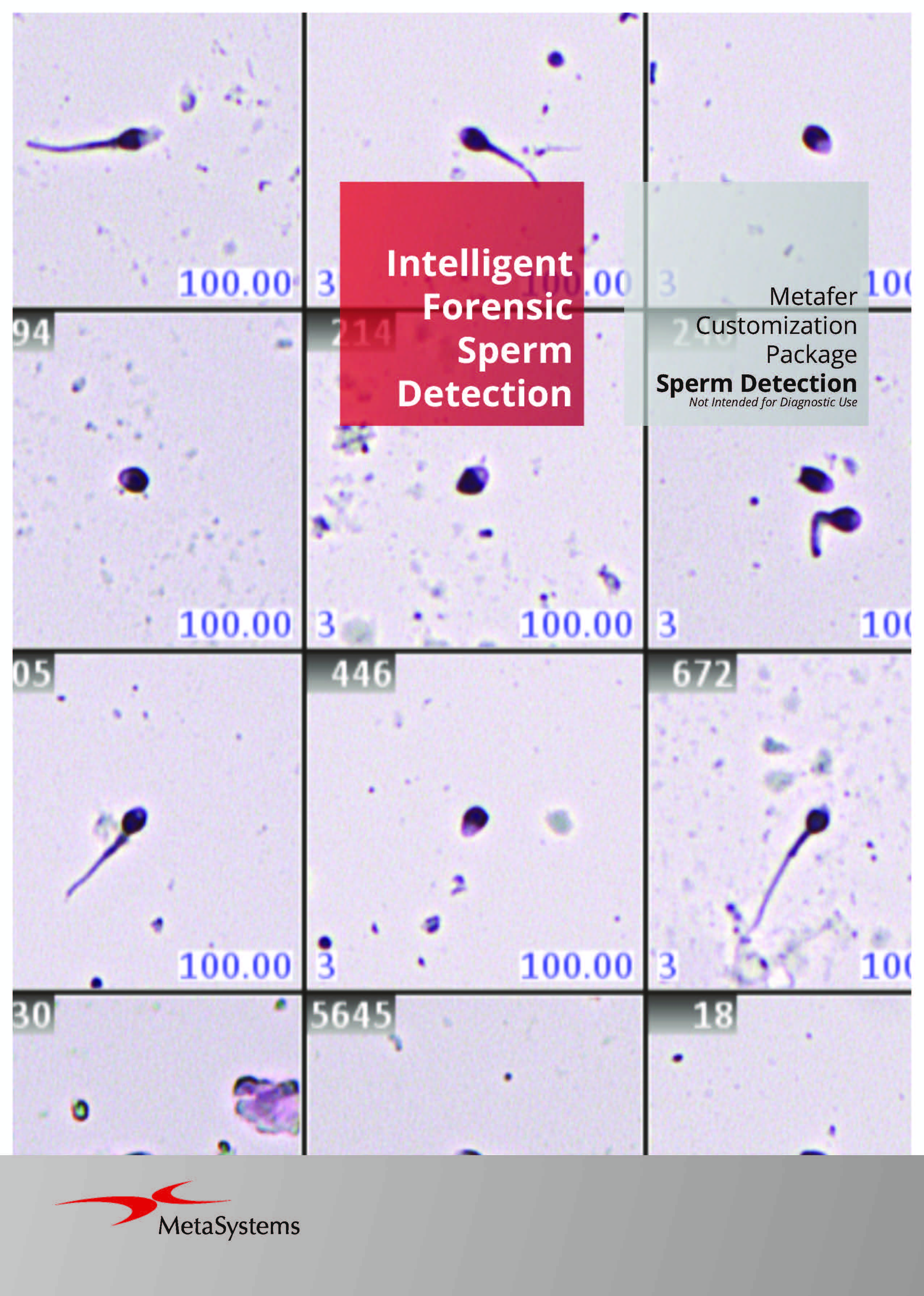
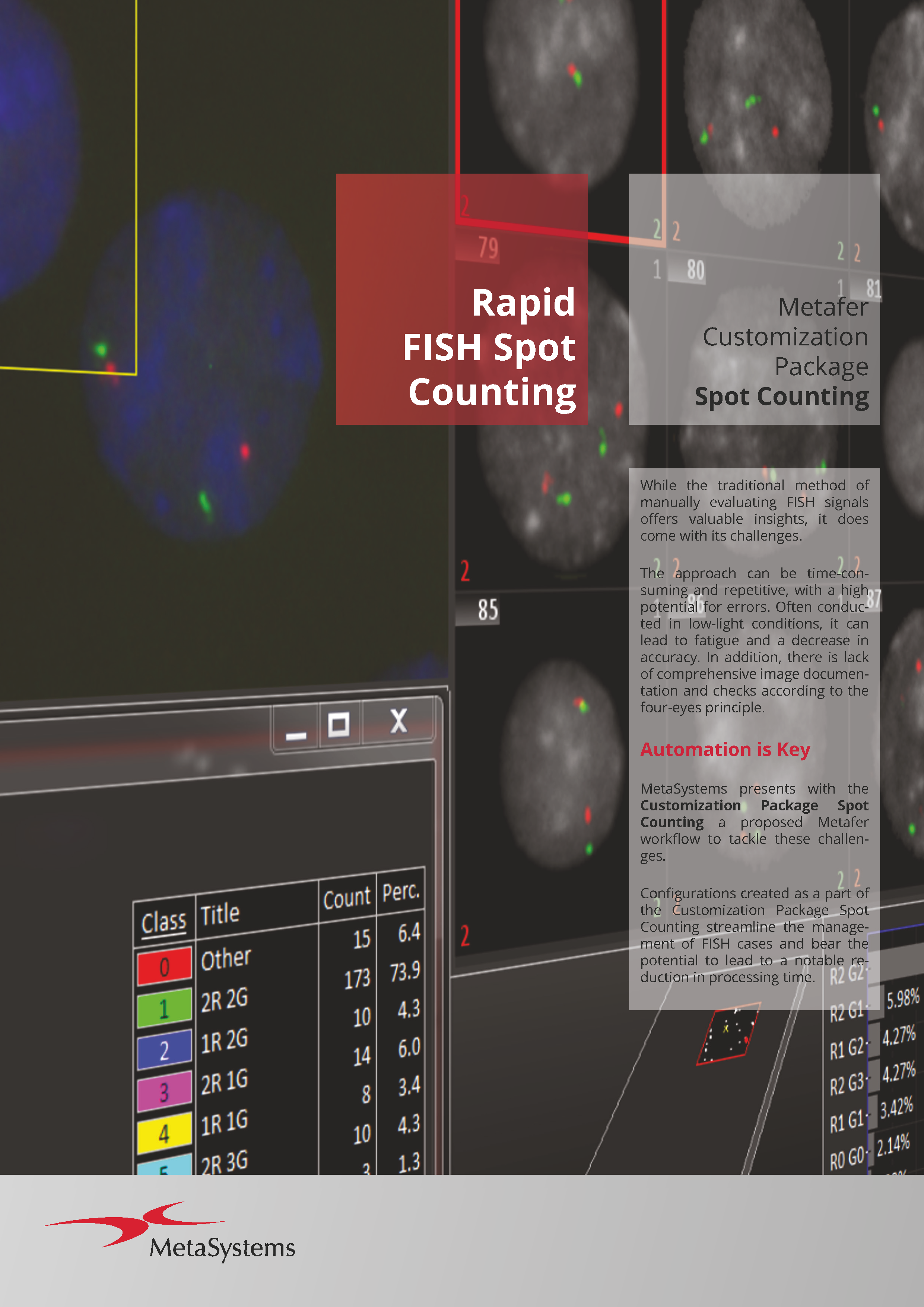
The flexible smart scanning facilitated by Metafer software suggests numerous options, each tailored to specific hardware accessories and software requirements for various customizations. A variety of software modules and hardware options are at your disposal. Commonly, Metafer software-controlled integrated systems consist of one of four camera models and a motorized stage. A fully integrated system requires a motorized microscope. MetaSystems can incorporate the microscope into the installation, although the Metafer software can also be integrated with existing, compatible microscopes.
Metafer 4.3 and Ikaros 6.3 are classified as in vitro diagnostic medical devices (IVD) in the European Union in accordance with In Vitro Diagnostics Regulation (EU) 2017/746 or In Vitro Diagnostic Medical Device Directive 98/79/EC, respectively, and carry the CE label unless otherwise indicated. Use all MetaSystems IVD products only within the scope of their intended purpose.
MetaSystems products are used in many countries worldwide. Depending on the regulations of the respective country or region, some products may not be used for clinical diagnostics.
Some hardware components supplied by other manufacturers are not included in MetaSystems IVD products and are therefore not IVD medical devices.
Please contact us for further information.